The Theory of Thermodynamics in Physics
The Theory of Thermodynamics: The Heartbeat of Physics:
Thermodynamics is one of the most curious branches of physics. It is basically the study of energy, heat, and work. This may sound complicated, but thermodynamics is most definitely an everyday part of your world – whether that is your morning coffee cooling, a car engine running, or just your body internally working to stay warm.
In this blog post, I will try to break this subject down simply. We will tell you the basic laws, and then I will provide you with some real examples, and then conclude by discussing why thermodynamics is important in science and technology.
What is Thermodynamics?
The term thermodynamics is derived from two Greek words: therme (heat) and dynamis (power). Therefore, thermodynamics is literally the study of the conversion of heat into power or other forms of energy.
More technically, thermodynamics deals with the relationships between heat, work, temperature, and energy and helps us develop an understanding of how energy moves and changes forms.
Why is Thermodynamics Important?
Thermodynamics is the basis for many things we use on a daily basis. Here are a few of those examples:
Engines: Car engines, airplane turbines, and rocket engines all rely on thermodynamics.
Home draft unit: Refrigerators and freezers, air conditioning units, and heaters are all home draft units that utilize thermodynamics.
Human systems: Your body is a small thermodynamics system which uses food (chemical energy) and converts that into motion, heat, and work.
Electricity Generation: Thermodynamic cycles create electricity in coal, nuclear, and/or solar power plants.
Modern technologies would not exist without thermodynamics.
The Four Laws of Thermodynamics:
There are four main laws of thermodynamics. They are usually numbered from zero to three. Let’s break them down simply:
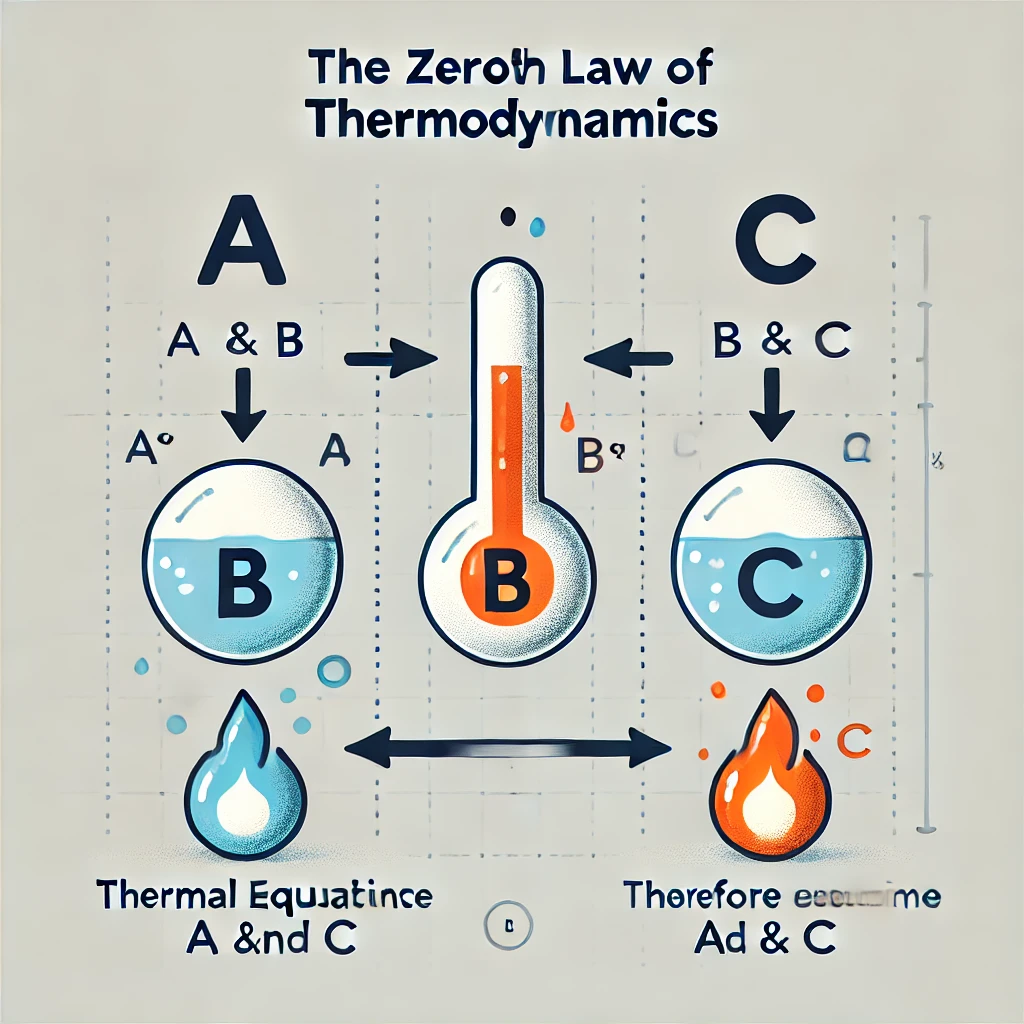
Zeroth Law of Thermodynamics:
If object A is in thermal equilibrium with object B, and B is in thermal equilibrium with object C, then A is also in thermal equilibrium with object C.
Although the law may seem to be a little strange, the transitive property of thermodynamic equilibrium is very important, since it also helps define what we mean by “temperature.” Essentially, if the three objects are at the same temperature, then there is no net heat transfer between the three objects.
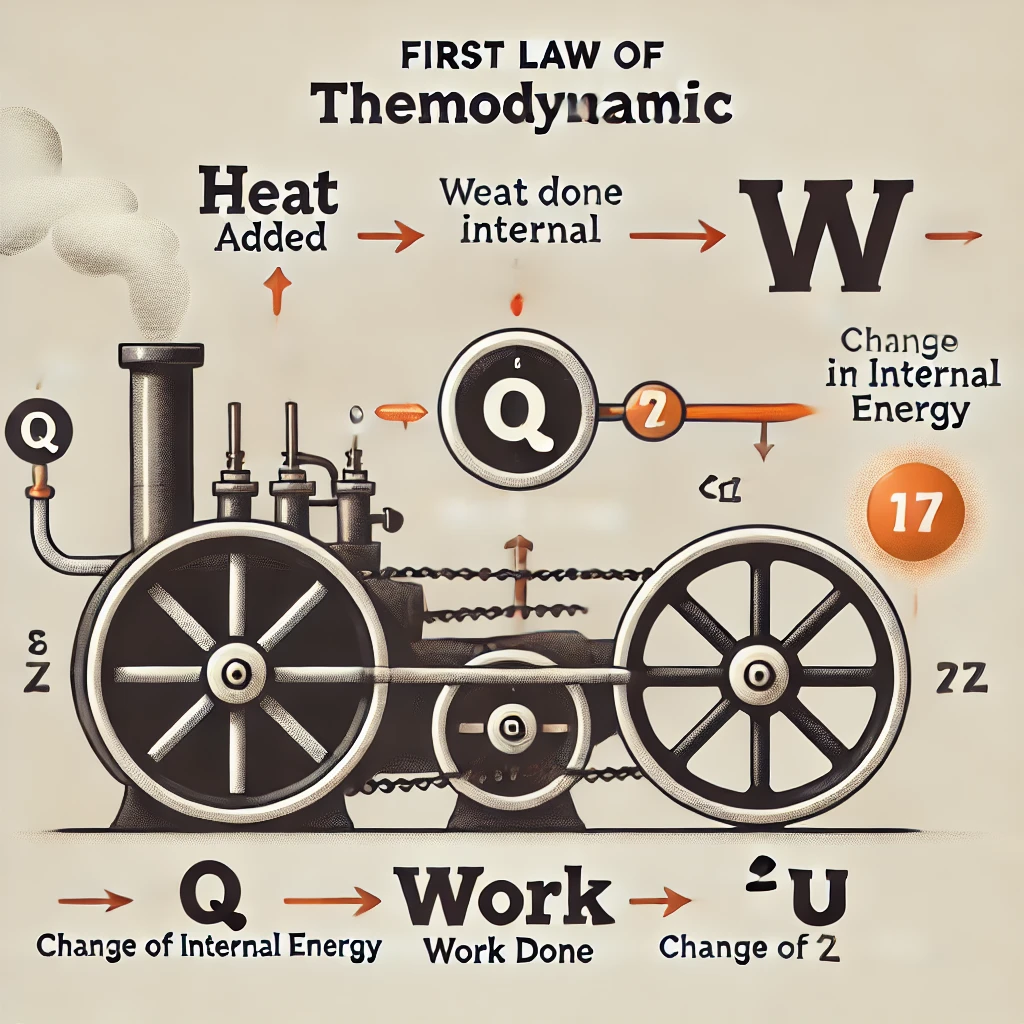
First Law of Thermodynamics:
Energy cannot be created or destroyed. Energy can only change forms.
This is another version of the Law of Energy Conservation. For example, if you heat a pot of water, the heat energy that was added to the water increases the temperature of the water. The heat energy you added to the pot may have also transferred some energy to the air surrounding it, however, the total energy still remains constant.
The First law can also be expressed in a formula:
ΔU = Q – W
Where:
ΔU is the change in internal energy;
Q is the heat addition to the system; and
W is the work done by the system.
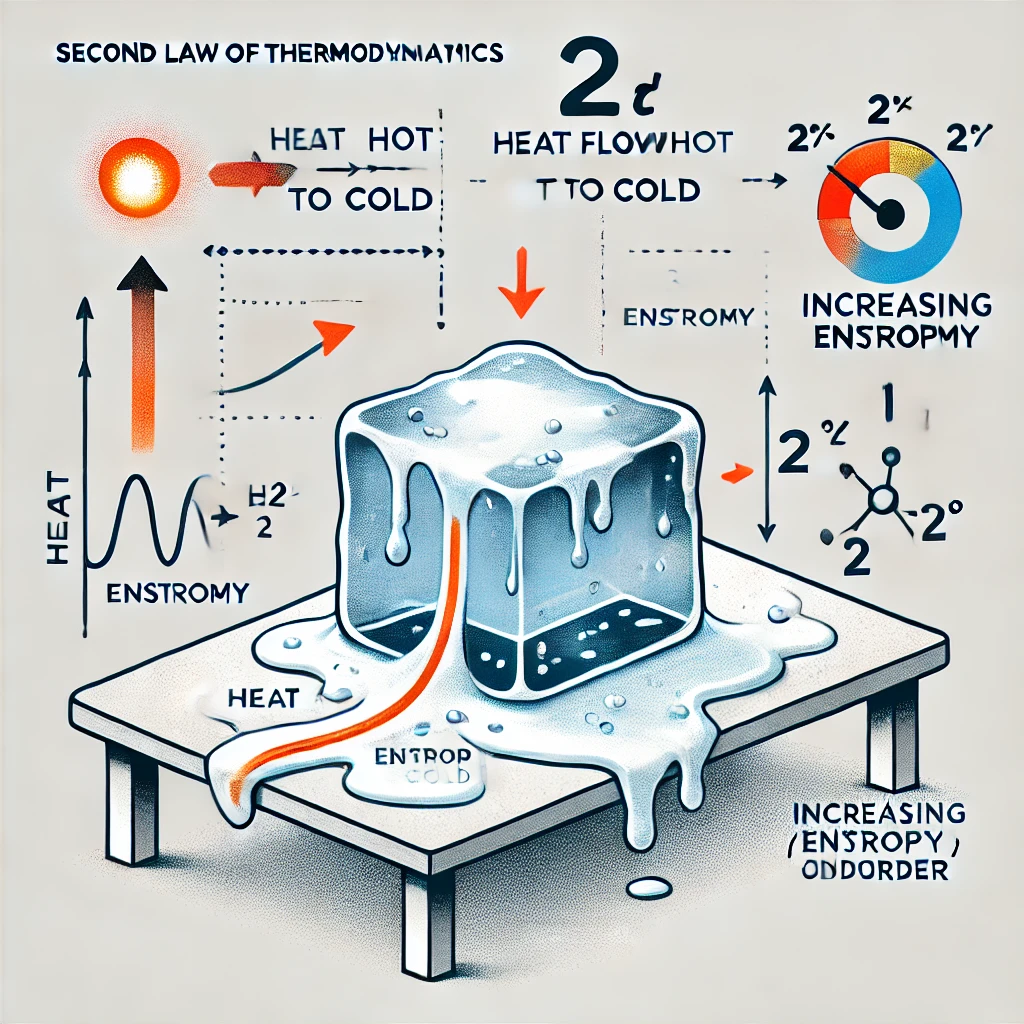
Second Law of Thermodynamics:
Heat moves in one direction: hot to cold, never cold to hot—unless you do work.
This law introduces entropy, a way of measuring disorder. In general, things in nature move from order to disorder. For example, if I opened a perfume bottle in a room, the scent expands and experiences disorder as it spreads. The scent never goes back to the bottle by itself without some outside intervention.
This was law explains why machines are not 100% efficient and why perpetual motion machines (machines that can run forever without an input of energy) are impossible.
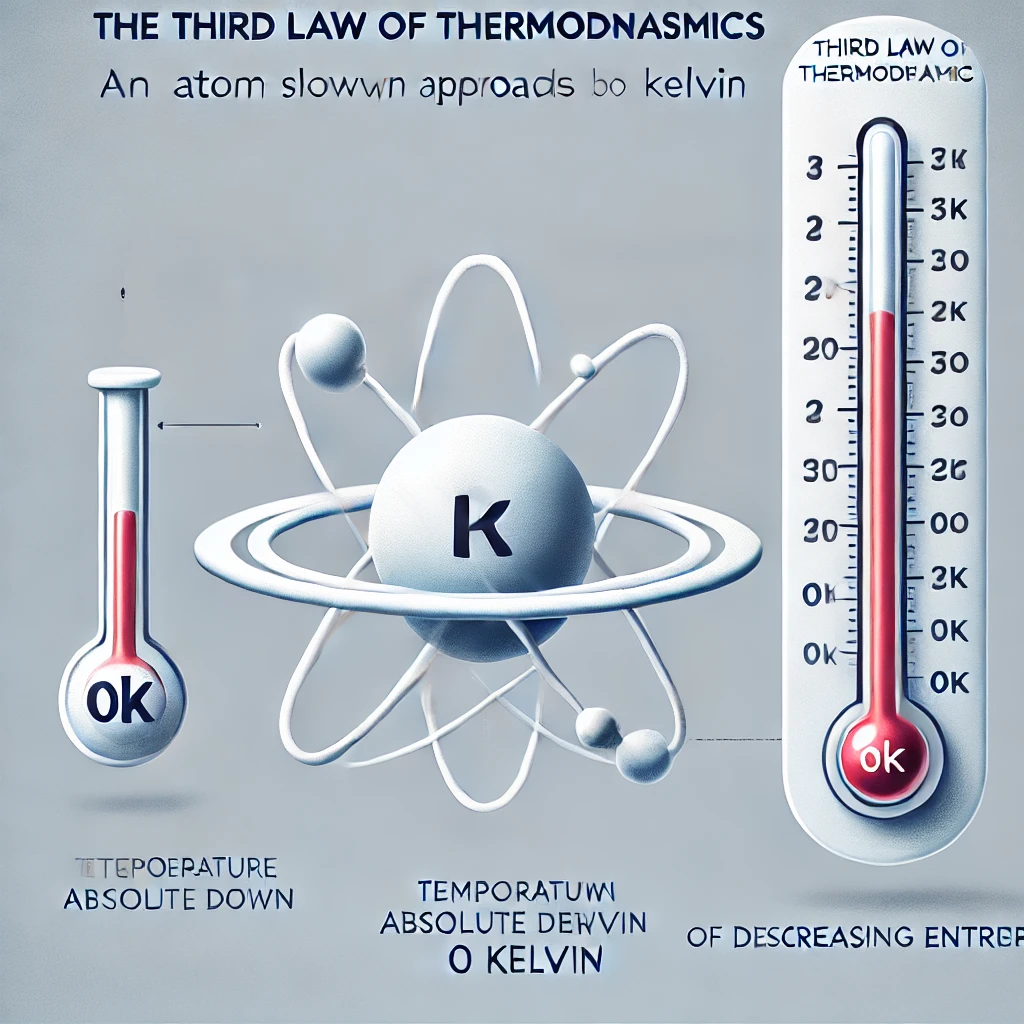
Third Law of Thermodynamics:
As the temperature of a system approaches absolute zero, the entropy (or disorder) of the system approaches a minimum.
Absolute zero also known as zeroth Kelvin or -273.15°C, is the coldest possible temperature, where nothing moves—not even atoms. This law is telling us that we can’t reach absolute zero; however, we can approach it.
Basic Thermodynamic Terms
The following are some important terms that will be beneficial for you in understanding thermodynamics:
System: This is the part of the universe we are studying (e.g., a gas in a container).
Surroundings: This is everything that is not the system.
Heat (Q): This is energy that is transferred due to a temperature difference.
Work (W): This is energy used to move an object.
Internal Energy (U): This is all of the energy contained in the system.
Entropy (S): This is the measure of disorder or randomness.
Types of Thermodynamic Systems:
There are three types of thermodynamic system:
1. Open System:
Matter and energy can both enter and/or leave. (for example, the water in a pot as it boils with the lid off)
2. Closed System:
Energy can enter and/or leave, but matter cannot. (for example, a sealed container of food being heated up)
3. Isolated System:
Neither energy nor matter can enter or leave. (for example, an ideal thermos flask)
Real-Life Examples of Thermodynamics
Let’s examine a few examples where thermodynamics is happening:
1. Vehicle Engine (Internal Combustion Engine)
Fuel combusts inside an engine, turning chemical energy into heat. Then that heat will do work on pistons which moves your vehicle. Some of that energy is lost as heat in surrounding air and exhaust.
2. Refrigerator
A refrigerator takes heat from the food inside it and transfers it out into the room. This is somewhat of a challenge because heat naturally flows from warm to cold (which is related to the second law of thermodynamics) and so it requires electricity to do this work—just like the second law suggests!
3. Steam Tubing
Water is boiled to generate steam in a power plant. The steam turns the turbines used to generate electricity. All of this uses thermodynamic cycles, especially, the Rankine cycle.
4. Human Body
When you consume food, your body breaks down the food to get energy. That energy keeps you warm, helps you move, and keeps your brain powered—all examples of energy transformations.
Thermodynamic Cycles
Thermodynamic processes often repeat in cycles. The Carnot cycle is a thermodynamic cycle that illustrates the maximum possible efficiency of an engine. While no engine of the Carnot cycle exists in reality, it is a useful ideal to compare against.
Biological science, as mentioned earlier, is involved in thermodynamics.
The Future of Thermodynamics
This is not the end of thermodynamics since thermodynamics is not just about engines and refrigerators; modern science has moved out of simple thermal and mechanical engines and moved to more interesting and useful science by making use of thermodynamic processes, for example:
One field of science that relies very heavily on thermodynamics is in climate science: how heat and energy moves throughout the Earth’s atmosphere.
Another field of science that relies on thermodynamics is in renewable energy: engineers might use thermodynamic principles and design to develop better solar panels, wind turbines, etc.
A growing field is called quantum thermodynamics, which is studying the thermodynamic principles of thermodynamics based on individual atoms!
Some researchers have even been leveraging thermodynamics to study black holes and theorizing about the beginning of the universe!
Conclusion:
Thermodynamics is the science of energy and energy transformations. It provides us with the framework to understand what is happening when something gets hot (the transfer of heat to an object) or when work is done (the transfer of energy to an object) and what the perspective limits are for machines in real life (the laws of thermodynamics). Thermodynamics is simple, immediately useful to our daily life and has massive implications for all of science and engineering.
Thermodynamics helps explain the workings of our universe – one degree at a time – from the warm cup of tea to the distant stars above.
FAQ -The Theory of Thermodynamics in Physics
1. What is thermodynamics in simple words?
Thermodynamics is the study of the transfer and conversion of heat and energy into work. It is the reason we can understand how engines work, refrigerators, and even our own bodies.
2. Why is thermodynamics relevant in everyday life?
Thermodynamics is useful because it allows us to design machines, such as engines, refrigerators, air conditioners, and power plants. It is also useful in medicine, climate science, and even cooking!
3. What are the four laws of thermodynamics?
1. Zeroth Law – Describes temperature and thermal equilibrium.
2. First Law – Energy cannot be created or destroyed, only transformed.
3. Second Law – Heat flows spontaneously from hot to cold and not the other way.
4. Third Law – When temperature approaches negative absolute zero disorder (entropy) approaches a minimum; absolute zero is unattainable within a finite number of steps.
4. What is entropy in layman’s terms?
Simply put, entropy is a measure of disorder or randomness. More disorder means higher entropy. For example, when an ice cube melts, the molecules move from ordered (solid) to disordered (liquid) so entropy increases.
5. What is an example of thermodynamics in everyday life?
A great example is a cup of hot tea cooling down. The hot tea’s heat moves into the surrounding air until everything reaches the same temperature. This is thermodynamics in action.

CreatBot D600 Pro 2 is a state-of-the-art 3D printing device designed for professionals requiring accuracy, reliability, and versatility in 3D printing. As part of the D600 series, it incorporates a large build volume, advanced dual extrusion technology, and top-tier features suitable for industrial use and complex materials.
Overview of the CreatBot D600 Series
The CreatBot D600 and D600 Pro establish benchmarks for large-scale 3D printers solutions. With a build volume of 600 ? 600 ? 600 mm, these industrial 3D printers cater to a wide range of industrial 3D printing demands, from big model prototyping to end-use production. The D600 pro series and the latest D600 Pro 2 introduce further enhancements in performance and material compatibility.
Key Features and Advantages
Large Industrial Build Volume
Build volume: 600 ? 600 ? 600 mm
Ideal for large-scale 3D printer projects and industrial 3D printing
Supports technical materials and intricate models
Dual Extrusion and High-Heat Printing
4th generation dual 1.75mm extruders for multi-material printing
Right and left extruder design for flexible printing
Supports high performance 3D materials, including PLA filament, nylon, carbon fiber, and more
Maximum extruder temperature: up to 420°C (high-heat)
Heated build chamber for premium applications
Accuracy, Speed & Dependability
Professional 3d print quality with accurate layer resolution
Advanced motion system for fast printing and robust performance
Consistent print speed up to 120 mm/s
Reliable operation for continuous industrial use
Supported Materials and Filaments
Wide Filament Compatibility
Works with a broad spectrum of filament: PLA, ABS, PC, PETG, PVA, nylon, carbon-fiber, and more
Designed for engineering-grade materials and functional prototyping
Advanced dual extruder 3d printer enables multi-material and soluble support printing
Applications: From Prototyping to Production
The CreatBot D600 Pro 2 and D600 Pro serve a diverse set of applications:
Rapid prototyping and large scale 3D printing models
Functional parts for automotive, aerospace, and engineering
Tooling, jigs, and fixtures for industrial production
Art, architecture, and creative projects requiring large industrial 3D printing
Technical Specifications
Model: CreatBot D600 Pro 2, D600 Pro, D600
Build volume: 600 ? 600 ? 600 mm
Extruders: Dual extruder, 4th generation 1.75mm dual extruders and hotends
Maximum extruder temperature: 420°C
Bed temperature: up to 100°C
Filament diameter: 1.75 mm
Layer resolution: 0.05 – 0.3 mm
Supported materials: PLA, ABS, PC, PETG, PVA, nylon, carbon fiber, engineering-grade materials
Print speed: up to 120 mm/s
Enclosure: Heated, for improved material properties
Interface: Touchscreen interface
Supported file types: STL, OBJ, AMF
Comparing D600 Models
Key Differences
D600: Entry-level industrial large scale 3d printer for basic applications
D600 Pro model: Enhanced with heated chamber, auto bed leveling, and wider material support
D600 Pro 2 model (professional version): Adds higher printing speed, improved reliability, and HS (high speed) configuration
Other CreatBot Models
CreatBot D1000 for even larger build volumes
CreatBot 3D printer includes industrial and professional 3d printer solutions
FAQ
What materials can the CreatBot D600 Pro 2 print?
The D600 Pro 2 is compatible with a wide range of filament including PLA, ABS, PETG, PC, nylon filament, carbon-fiber, and other engineering-grade materials.
What is the maximum build volume of the D600 Pro 2?
The build volume is 600 ? 600 ? 600 mm, supporting large model and industrial 3d printing needs.
Dual Extruder and High-Temp Support on D600 Pro 2
Yes, it is equipped with dual extrusion technology and reaches up to 420°C for high-temperature printing process.
What differentiates the D600 Pro 2 from the D600 Pro?
The D600 Pro 2 offers higher printing speed, improved reliability, and the new HS (high speed) option.
Summary
The D600 Pro 2 and the D600 Pro set the benchmark in the industrial 3D printer category. With exceptional build volume, robust dual extruders and hotends, compatibility with technical materials, and top performance across applications, they empower businesses and engineers to achieve new heights in industrial 3d printing.
nylon
large format 3d printer
dual extruder
creatbot d600 pro industrial
Pingback: History of Hindu Religion
Pingback: How Does a Drone Work? - Topbloggs